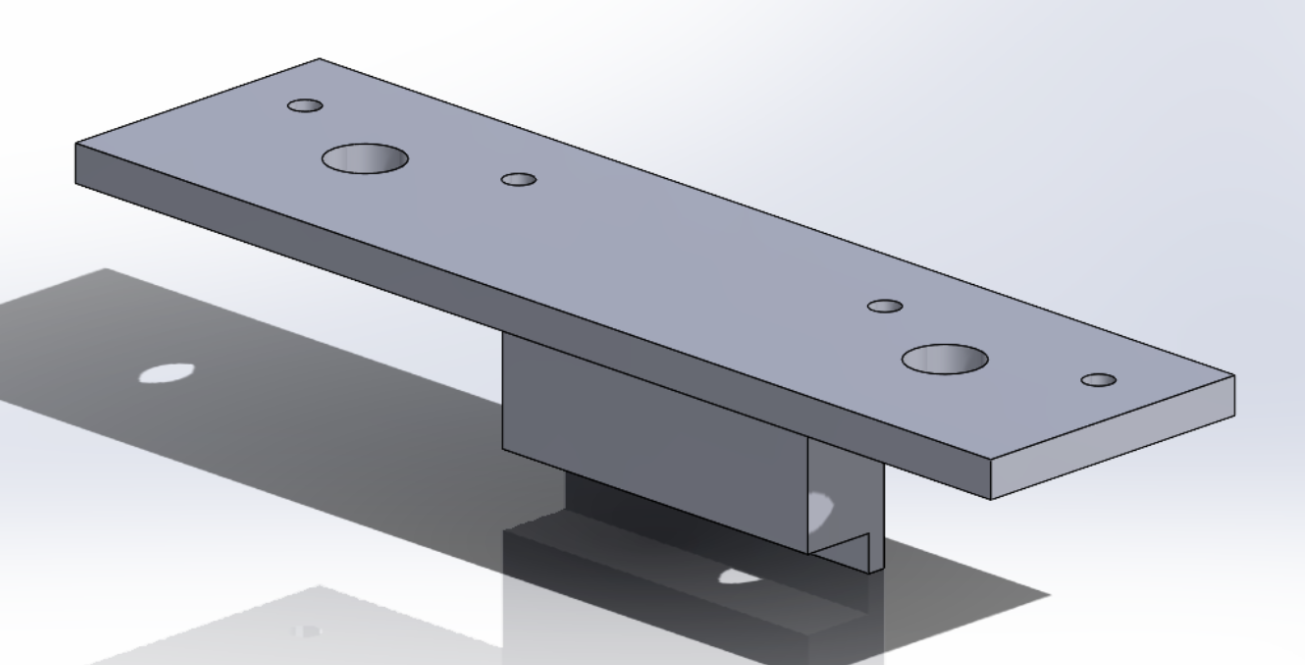
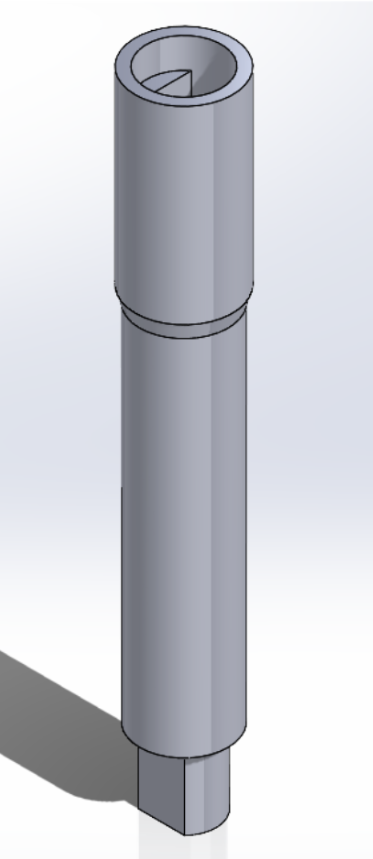
Motor Holder
- Material:PLA
- Stepper motor holders pass through the knob and fixate the motors position.
Learn the details of the assembly of the final product.
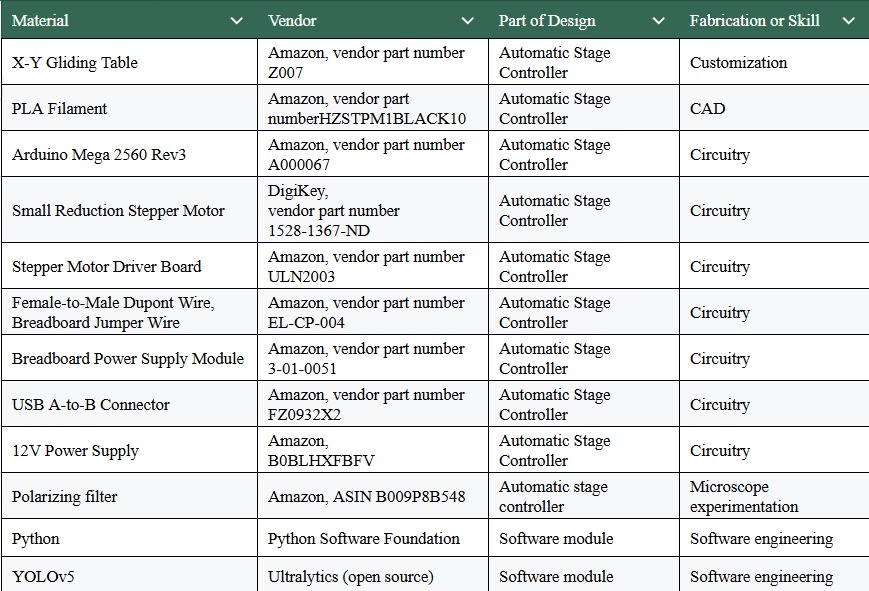
We 3D printed the following series of components.

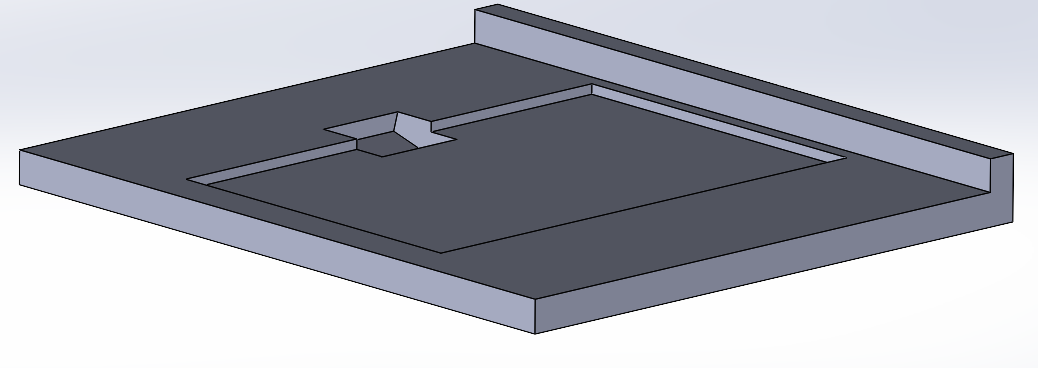
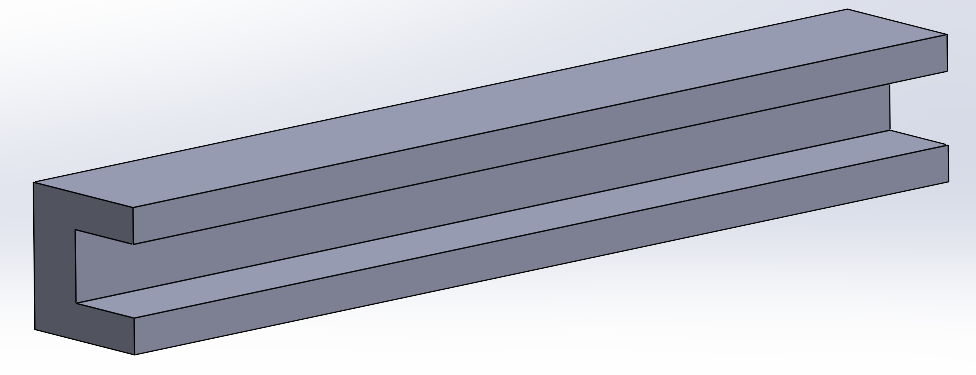
Since this project is not an actual product but rather a way to optimize a process through an equipment, the manufacturability relies on how easy it is to replicate the equipment and perform the right maintenance.